Description:
The MR Detection for Alk-Phos, Anti-Polyvalent provides unmatched sensitivity with incubation times of 10 minutes each for the Link Antibody and Enzyme Label.
Contains:
- One container of Protein Block.
- One container of Anti-Polyvalent.
- One container of Alkaline Phosphatase.
Uses/Limitations:
- Not to be taken internally.
- For In Vitro Diagnostic Use.
- Histological applications.
- Do not use if reagents become cloudy.
- Do not use past expiration date.
- Use caution when handling reagents.
- Non-Sterile.
Control Tissue:
Any well-fixed tissue section.
Precautions:
Avoid contact with skin and eyes.
Harmful if swallowed.
Follow all Federal, State, and local regulations regarding disposal.
| Species of Origin: | Goat |
| Antigen Specificity: | Anti-Mouse, Anti-Rabbit |
| Preadsorbed Against: | Human |
| Enzyme Conjugate: | Alkaline Phosphatase |
| Chromogen Substrate: | None Provided |
| Storage: | Store at 2-8°C. |
Procedure:
1. Rehydrate tissue slides.
2. In a glass or plastic (Autoclavable) Coplin jar, add retrieval solution of choice.
3. Submerge slides in retrieval solution and loosely cap.
4. Add Distilled water to bottom of Autoclave or Pressure Cooker (about 1 inch deep in Pressure Cooker).
5. Place Coplin jar in Pressure Cooker or Autoclave.
6. Turn heat on and allow pressure to rise to 20-25 PSI.
7. Maintain pressure at 20-25 PSI for 5 minutes.
8. Turn off heat source and allow to cool.
9. When pressure has dropped to ambient, carefully remove lid or open door.
10. Using tongs, remove Coplin Jar and place on counter.
11. Once Coplin Jar cools to room temperature remove slides, rinse several times in buffer and proceed with staining as usual.
12. Rinse in distilled/DI water.
13. Rinse 3 times in buffer.
14. Apply Protein Block, and incubate for 5 minutes at room temperature to block nonspecific background staining. Note: Do not exceed 10 minutes or there may be a reduction in desired stain.
15. Rinse 1 time in buffer.
16. Apply primary antibody (Mouse or Rabbit) and incubate according to manufacturer’s protocol.
17. Rinse 3 times in buffer.
18. Apply Anti-Polyvalent and incubate for 10 minutes at room temperature.
19. Rinse 2 times in buffer.
20. Apply Alk-Phos and incubate for 10 minutes at room temperature.
21. Rinse 2 times in buffer.
22. Rinse 1 time in Distilled/DI water.
Mix Ember Red Concentrate with Ember Red Buffer (not included).
23. Combine 10µl of Ember Red Concentrate with each 1ml of Ember Red Buffer. Combined mixture may be used for up to two hours.
24. Apply adequate mixed reagent to cover tissue section completely and incubate for 5-10 minutes.
24. Rinse 1 time in Distilled/DI Water.
25. Apply Ember Red Chromogen/Substrate mixture and incubate for a second 5 minute period.
26. Rinse 3 times in Distilled/DI water.
27. Apply Hematoxylin for Automation (HAQ) and incubate for 1 minute.
28. Rinse 3 times in distilled water.
29. Apply Bluing Reagent (BRT) and incubate for 5-10 seconds.
30. Rinse immediately in distilled or deionized water.
31. Quickly dehydrate in alcohol and clear in xylene or substitute.
Note: Alcohol and Xylene can cause chromogen to leach from tissue over extended periods of time.
32. Coverslip using a permanent mounting media.
-Troubleshooting Guide-
Overstaining:
1. Concentration of the primary antibody was too high or the incubation time was too long.
2. Temperature during incubation was too high.
3. Incubation times were too long.
Non-Specific Background Staining:
1. Rinsing between steps was inadequate.
2. Tissue was allowed to dry with reagents on.
3. Folds in tissue trapped reagents.
4. Antigen migrated in tissue.
5. Excessive tissue adhesive on slides.
6. Inadequate blocking with protein block.
Weak Staining:
1. Primary antibody concentration was too low or incubation time was too short.
2. Reagents are past their expiration date.
3. Inadequate removal of wash buffer between steps, resulting in dilution of reagents.
4. Room temperature was excessively cool.
5. The primary antibody does not recognize an antigen that survives fixation and embedding in high enough amounts.
6. Excessive incubation with protein block (Super Block or normal serum).
No Staining:
1. Steps were inadvertently left out.
2. There is no antigen in the tissue.
3. The primary antibody is not of mouse or rabbit origin.
4. Chromogenic substrate has been replaced with another that is not intended for use with alkaline phosphatase.
5. One or more components of the kit have been inactivated.
References:
1. Hsieh MH, Jan RL, Wu LS, Chen PC, Kao HF, Kuo WS, Wang JY. Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. Journal of Molecular Medicine. 2018 Jan 1;96(1):39-51.
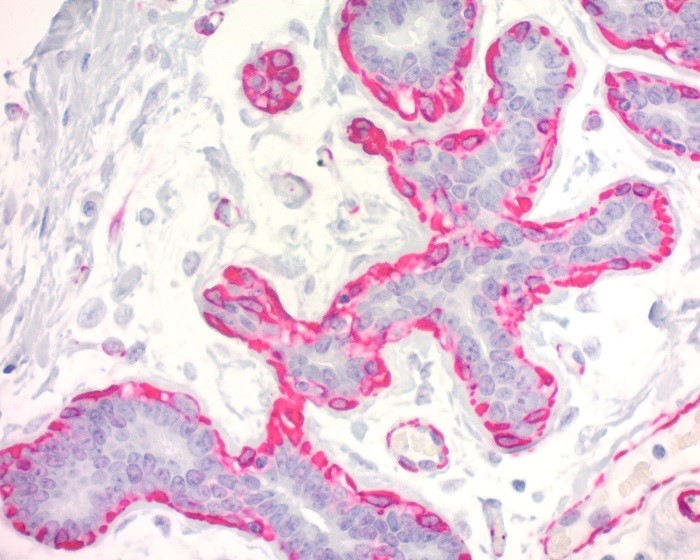
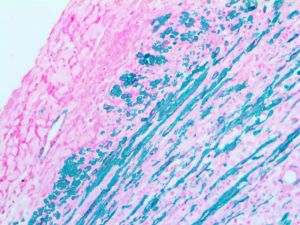
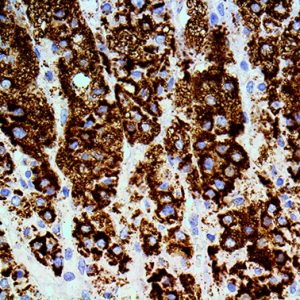


Reviews
There are no reviews yet.